Microscope slides play a crucial role in examining single-celled organisms and observing small plants and organisms up close. There are primarily two methods for preparing slides: dry mounts and wet mounts. Each method serves its purpose in mounting different types of cells. When dealing with pale or translucent specimens in wet mounts, staining may be necessary to enhance visibility under the microscope.
Procedures
Preparing a Dry Mount

Choose a clean slide. Hold the slide against a light source to ensure it is free from smudges and dirt. Most microscope slides have a flat, rectangular shape with clear surfaces allowing light to illuminate the sample. Any contamination, including fingerprints, should be removed by washing the slide with liquid soap and water, followed by drying with a clean cloth (avoiding tissues or paper towels to prevent leaving lint).
- If contamination is found on the slide, including fingerprints, wash it quickly with liquid soap and water. Dry the slide using a clean cloth. Do not use tissues or paper towels, as these can leave lint behind.

Examine the specimen to determine if slicing is necessary. The specimen should be sufficiently translucent for light to pass through. If light cannot penetrate the specimen fully, it won’t be visible through the microscope’s eyepiece.
- Some specimens, such as a strand of hair or an insect wing, are naturally thin and translucent and may not require slicing with a razor blade.

Trim a thin section from the specimen. Use a razor blade to carefully slice your specimen into a thin, translucent piece. Dry mounts are the easiest to prepare as they do not involve any liquid between the slide and specimen. Dry mounts are suitable for examining samples that won’t dry out easily. Common materials for dry mounting include:
- Cork or balsa wood.
- Flower petals or leaves.
- Insect legs or wings.
- Hair, fur, or feathers.

Position the specimen on the slide. Use forceps to place the thin slice of your specimen delicately onto one side of the slide. If using a concave slide (with one side dipping down), center the specimen in the concave area.
- Opt for a concave slide if there's a risk of the specimen rolling off a flat slide. For instance, use a concave slide when preparing a curled flower petal prone to rolling.
- Flat slides suffice for other specimen types.

Apply a cover slip over the specimen. The cover slip prevents the specimen from slipping off the slide and shields it from accidental contact if the microscope lens is lowered too far.
- Cover slips are thin, transparent pieces of glass or plastic, typically about 3⁄4 inch (1.9 cm) in width and length.
- Your prepared slide is now ready for examination under a microscope.
Preparing a Wet Mount

Add 1 water drop onto the slide. Use an eyedropper to carefully place a single drop of water at the center of a flat or concave slide. This water drop is essential for creating a wet mount, ensuring that the sample specimen remains moist and retains its shape. It also serves to preserve living specimens, such as single-celled organisms.
- If you wish to create a permanent slide with deceased organic material, you can substitute the water droplet with a thin layer of clear nail polish.
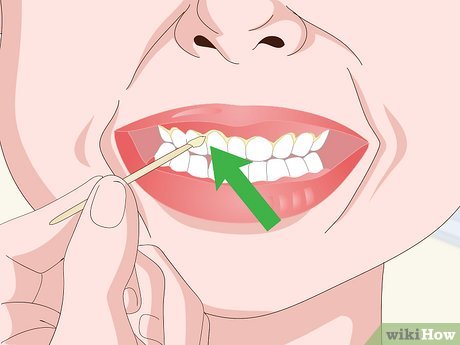
Extract a portion of the wet sample specimen. Wet mount specimens typically consist of wet or living organic material. Use a razor blade or toothpick to collect a small amount of your wet specimen. Common materials for wet mount slides include:
- Cheek cells or tooth plaque (scraped from your mouth using a toothpick).
- A thin slice of a plant stem (cut with a razor blade).
- For single-celled organisms like amoebas or paramecia, tweezers are ineffective. Instead, use a clean eyedropper to collect a few drops of the water containing the organisms or algae.

Position the sample specimen in the water drop. Depending on the nature of your specimen, use forceps, tweezers, or a toothpick to transfer it onto the slide. Center the specimen within the water droplet, ensuring it remains suspended in the liquid.
- If using an eyedropper for single-celled organisms, add 1 or 2 drops to the existing water drop on the slide.

Place a cover slip over the wet specimen. Hold the cover slip at a 45° angle and lower one edge next to the specimen on the water drop. Gradually lower the opposite side until it rests flat on the specimen. Observe the water drop(s) spreading beneath the cover slip until they reach its edges.
- Avoid tapping or pressing the cover slip once it's in position to prevent squeezing out the sample specimen and water from the slide.
Staining Cellular Specimens

Position a paper towel against one edge of the cover slip. Place the towel gently against the edge of the slip, ensuring the material beneath remains undisturbed. The absorbent towel will draw water from beneath the cover slip, facilitating the spread of the staining agent onto the specimen.
- Staining is beneficial for enhancing visibility of pale or colorless wet-mounted specimens, such as cross-sections of colorless plant stems, by improving their shape and texture under the microscope.
- Typically, staining is performed after initial examination of the wet specimen on an unstained slide. The slide may already be prepared even if unstained.
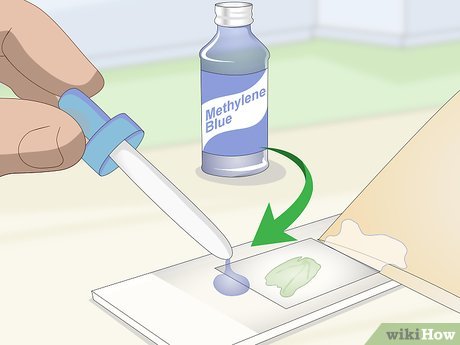
Apply 1 drop of iodine or methylene blue on the opposite side of the cover slip. Use an eyedropper to dispense the staining chemical next to the cover slip on the microscope slide, being careful to release only a single drop. Excess staining agent may overflow from the slide.
- Iodine or methylene blue can be acquired from educational or biology supply stores.
- An alternative method is to add the staining agent drop to the water on a wet-mounted slide during initial preparation, eliminating the need for a paper towel.

Allow the staining agent to diffuse under the cover slip. As the paper towel absorbs water from one side, the staining agent will gradually seep under the cover slip. This process may take up to 5 minutes for complete saturation of the specimen with iodine or methylene blue.
- Once the staining agent permeates entirely under the cover slip, the specimen is fully stained.

Remove excess staining agent with a fresh paper towel. Wipe the slide's surface clean to prevent any spills. Your stained wet-mounted slide is now ready for observation under the microscope.
Helpful Hints
Cautions
- Handle microscope slides and covers with caution due to their small and fragile nature. Avoid dropping or scratching them, and always place them on clean surfaces.
- Remember that iodine and methylene blue are toxic and should not be ingested. They can also stain skin temporarily and clothing permanently, so wear protective clothing when handling these chemicals.
Essentials
- Microscope
- Box of microscope slides
- Razor blade (optional)
- Toothpick (optional)
- Forceps
- Slide covers
- Eyedropper
- Iodine or methylene blue
