While fire typically emits yellow and orange hues, adding certain chemicals can change the flames to an extraordinary green. To achieve this effect, mix common household items containing copper or boron, such as boric acid, Borax, and copper sulfate, with methanol. Continue reading to find out how to create green flames, the chemistry behind the transformation, and whether green flames are safe to handle.
Creating Green Fire
Combine a spoonful of boric acid, Borax, or copper sulfate with an equal amount of methanol in a ceramic or steel dish. Ignite the mixture with a lighter or match to produce green flames. The presence of copper or boron in the fire causes the unique green color.
Procedure
Creating Green Flames with Boric Acid
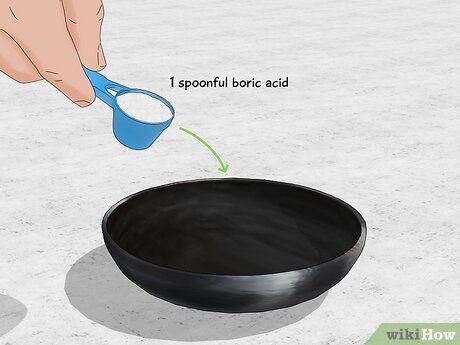
- Tip: If you can't find pure boric acid, look for a pesticide containing boric acid.
- Alternatively, you can use Borax instead of boric acid, as it also produces green flames.

- Tip: If you can't find pure methanol, use an antifreeze product that contains methanol, such as HEET.
- Methanol is typically used in engines or as antifreeze for cars.

- As the methanol burns, the flames will typically become smaller and dimmer.
- For more green flames, extinguish the fire, add more methanol, and ignite again. Since boric acid does not burn, no additional boric acid is needed.
- Warning: Only light the fire outside or in a well-ventilated area. Borax and boric acid can irritate the respiratory system and cause coughing. Methanol also emits CO2, which can be harmful in excessive amounts.
Creating Green Flames with Copper Sulfate
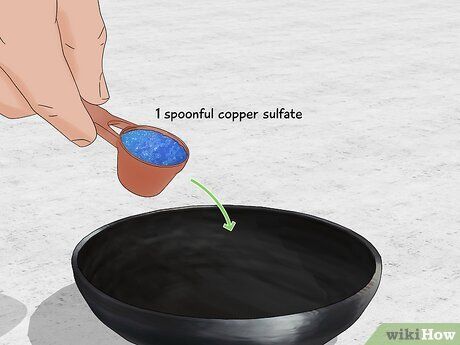


- Once the flames begin to fade, extinguish the fire and add more methanol to keep the flames green. The copper sulfate will not burn away.
- Warning: Only light the fire outside or in a well-ventilated space. Copper sulfate may cause eye irritation. Methanol burns produce CO2, which can be dangerous in high concentrations.
Which chemicals create green flames?
- The copper in copper sulfate is responsible for the green color in the flames.

- Both boric acid and Borax contain boron, which explains why they produce green flames.

- Warning: Burning pressure-treated wood is hazardous. The chemicals it contains can release toxic fumes.
- Barium is a key ingredient in green fireworks.
Is green fire safe?

- To minimize the risk, make sure to create green flames outdoors or in a well-ventilated space.
Green Fire Temperature

- Sprinkle Borax, boric acid, or copper sulfate onto your bonfire to produce green flames as well.
Warnings
- Always exercise caution when creating a fire. Set up green flames in a fire-safe area, away from any flammable materials. Have a fire extinguisher or water nearby in case the flames get out of control.
- Make green flames outdoors or in a well-ventilated area. Handle boric acid, Borax, and copper sulfate with care, as these chemicals can be irritating to the eyes, skin, and respiratory system.
- Wear fire-resistant gloves and safety glasses to protect yourself from the flames and chemicals while making green fire.
- Keep boric acid, Borax, and copper sulfate out of reach of children and pets, as they can be harmful if ingested.
