At first glance, platinum, silver, or sterling silver may look almost identical to someone unfamiliar with these metals. However, with just a bit of practice, you'll be able to tell them apart in no time!
Examine the Jewelry
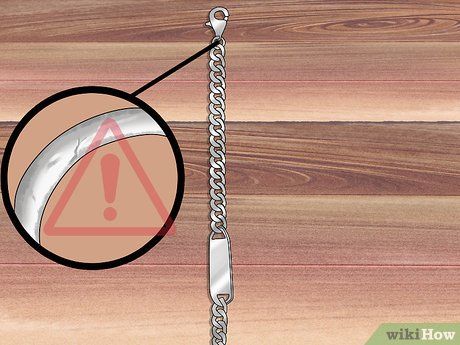
Look for a hallmark on the jewelry. The hallmark is usually etched into the metal surface. If the jewelry has a clasp, the mark may be found on the back of the clasp. It could also feature a small metal piece with the stamp. Finally, check the widest part of the jewelry.
- If the jewelry doesn’t have any markings, it’s likely not made of precious metal.

Consider both the color and weight of the jewelry. If you have the chance to compare platinum and silver, it becomes easy to tell them apart. Platinum has a much higher density than silver, so it will feel heavier. Additionally, platinum isn’t actually white—it’s more of a grayish color.

Look for silver jewelry marks. Some coins and jewelry will feature the number '999.' This mark indicates that the jewelry is made of pure silver. If the piece is marked with '925' followed by an 'S,' it’s sterling silver. Sterling silver is an alloy consisting of 92.5% pure silver and another metal, usually copper.
- For example, the 'S925' mark on jewelry indicates sterling silver.
- Pure silver jewelry is quite rare, as pure silver is soft and prone to damage.

Look for platinum identification marks. Platinum is a very rare and expensive metal, so all platinum jewelry will have a verification mark. Search for the word 'Platinum,' 'PLAT,' or 'PT' followed by the numbers '950' or '999.' These numbers represent the purity of the platinum, with 999 being the purest.
- For example, a genuine platinum piece might be marked 'PLAT999.'
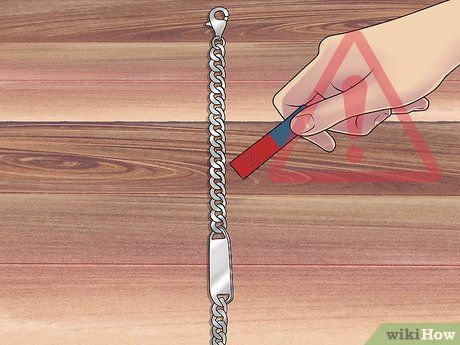
Test the jewelry with a magnet. Most precious metals are not magnetic, so if you bring a magnet near the jewelry, it should remain unaffected. However, if your platinum jewelry reacts to the magnet, don’t panic. Pure platinum is a soft metal and is often mixed with another metal to make it harder. Cobalt is a hard metal commonly used in platinum alloys and is slightly magnetic, so some platinum jewelry may respond to a magnet.
- Platinum/cobalt alloys are often marked 'PLAT,' Pt950, or Pt950/Co.
- The most common metal used to harden sterling silver is copper, which is not magnetic. If you have a sterling silver piece marked '925' but it attracts a magnet, take it to a trusted jeweler for authentication.
Using an acid testing kit

Use the acid testing kit to check jewelry that is difficult to identify. If you can't find any markings and are unsure about the origin of the jewelry, you can use an acid testing kit to determine the type of metal. Purchase the kit from online retailers or jewelry stores. The kit typically includes a honing stone and a few acid vials.
- Opt for a kit that can test both silver and platinum. The metal types are labeled on the acid vials.
- If the kit doesn't include gloves, it’s recommended to buy a pair. Acid can burn skin if it comes into contact with it.
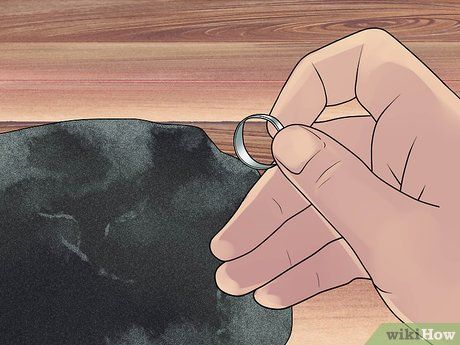
Rub the jewelry onto the stone. Place the black stone on a flat surface. Gently rub the jewelry on the stone in a back-and-forth motion to create a streak. Rub two or three streaks for different metals you'll test. For example, to test platinum, silver, and gold, create three streaks.
- Pick an inconspicuous area of the jewelry to rub on the stone. The stone surface will scratch and damage part of the jewelry.
- Place a cloth underneath the stone to protect the table surface.

Drop acid onto the metal streaks. Select an acid vial from the kit and carefully drop a small amount of acid onto one of the streaks on the stone. Make sure to keep the acids separate to avoid contamination that could affect the results.
- Most testing kits include acids specifically for silver testing. However, you can also use 18-karat gold acid to test for pure silver or sterling silver.
- Always wear gloves when handling acid.

Expert Advice: When testing platinum, rub the jewelry against a stone and then drop hydrochloric nitric acid onto it. If the streak remains intact, the jewelry is platinum. If the streak dissolves, it is not platinum.

Observe the reaction of the streaks to the acid. The reaction can occur within 1 second to 1 minute. If the streak dissolves, the jewelry is not the metal you are testing for. For example, if you apply platinum acid to the streak and it dissolves, the jewelry is not platinum; if the streak remains, it's pure platinum.
- If you use 18-karat gold acid to test silver, the streak will turn milky white, indicating the jewelry is pure silver or sterling silver.
- If you're still unsure about the result, retest for accuracy.
Use a direct testing solution on silver
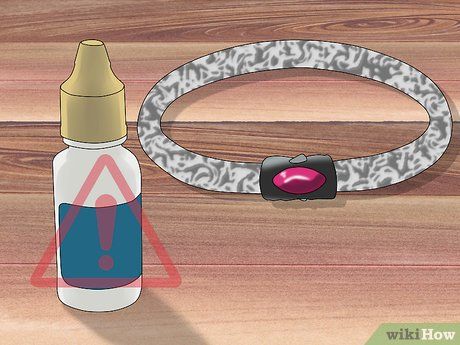
Use the silver testing solution on larger, sturdier jewelry. Avoid using acid on finely crafted pieces, as it will corrode any part it contacts. If you’ve purchased an acid test kit, use the silver testing solution included. Alternatively, you can buy silver testing solutions online or at jewelry stores.

Test the jewelry. Drop a small amount of silver testing solution onto the metal's surface. Choose a hidden area of the jewelry for testing. For example, if testing a wide bracelet, apply a few drops of acid to the inner side. For testing a thick, flat chain, apply acid to the back of a section of the chain.
- Wear gloves to protect your hands and place a cloth underneath to shield the work surface.
- Avoid dropping acid on the clasp or any important parts of the jewelry, as acid may damage small components.
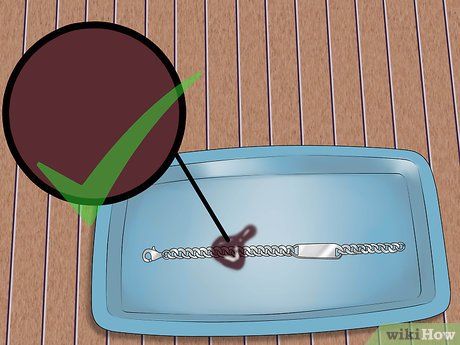
Observe the reaction. Initially, the acid will turn dark brown or clear, then change to another color. The new color indicates the purity of the metal. For instance, if the acid turns dark red or bright red, the metal contains at least 99% pure silver.
- If the solution turns white, the metal contains 92.5% silver, meaning it's sterling silver.
- If the acid turns a near-green color, the metal being tested is copper or another less valuable metal.

Rinse off the acid from the jewelry. Use a clean cloth to wipe away the acid and dispose of it. Then, rinse the jewelry under cold water to remove any remaining acid. Be sure to plug the sink to avoid losing the jewelry down the drain. Allow the jewelry to dry thoroughly in the air before wearing it again.
Test jewelry using hydrogen peroxide
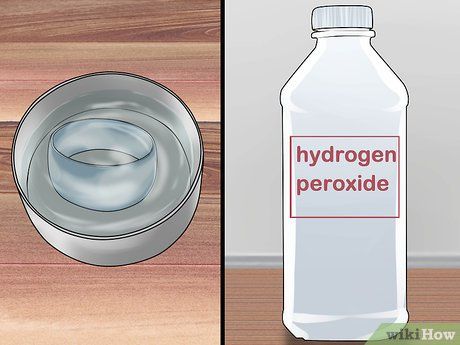
Soak the jewelry in hydrogen peroxide. First, pour hydrogen peroxide into a glass bowl or cup, then place the jewelry into the bowl. The jewelry should be fully submerged in the hydrogen peroxide. If not, add more hydrogen peroxide to cover it.
- You can purchase hydrogen peroxide at pharmacies.

Observe the reaction. Platinum is a strong catalyst for hydrogen peroxide. If the jewelry is platinum, hydrogen peroxide will begin to bubble almost immediately. Silver is a weaker catalyst. If you don’t see bubbles right away, wait for about 1 minute to see if small bubbles form around the jewelry.
- Hydrogen peroxide does not corrode or damage the jewelry.

Thoroughly clean the jewelry. Wash the jewelry under cold running water to remove any hydrogen peroxide. Be sure to block the sink drain to prevent the jewelry from falling into the pipes. Allow the jewelry to dry completely before wearing it.
Advice
- If you're still unsure about the authenticity of the jewelry, take it to a reputable jeweler for inspection.
Warning
- Keep acids and acid testing kits out of reach of young children. Acids can cause burns and can be fatal if ingested.

















