 Are you sure about how much you've had to drink before hitting the road, or are you simply estimating? Don’t worry, the authorities can assist you with that.
ANATOLII BOIKO/Stringer/Getty Images
Are you sure about how much you've had to drink before hitting the road, or are you simply estimating? Don’t worry, the authorities can assist you with that.
ANATOLII BOIKO/Stringer/Getty ImagesWe often hear about drivers involved in accidents who later face charges for drunk driving. News reports typically include the driver's blood alcohol content (BAC), such as 0.15, when the legal limit is 0.08. To understand how such accurate readings are possible, we need to ask: how does a breathalyzer work?
What do those numbers signify? And how do officers determine if a driver suspected of drinking is truly over the legal limit? Many law enforcement agents use breath alcohol testing devices to measure blood alcohol concentration (BAC) in suspected drunk drivers. In this article, we’ll explore the science and technology behind the Breathalyzer and other breath alcohol tests.
Alcohol Consumption Kills
It is crucial for public safety to remove drunk drivers from the roads. A 2023 statistic reveals that 37 people died in alcohol-related car crashes in the U.S., which translates to one death every 39 minutes. Alcohol impairment accounted for over 31% of all traffic fatalities.
Even drivers who pass roadside sobriety tests — such as touching their nose or walking in a straight line — may still be exceeding the legal blood alcohol limit and pose a danger. This is why police officers rely on advanced technology to measure alcohol levels in suspected drunk drivers and keep the streets safe.
If you are drinking, do not get behind the wheel. Breathalyzers provide highly accurate measurements of alcohol levels in a person’s breath. Plus, by taking a cab or using public transport, you can avoid the risks of drunk driving! If you're unsure about your ability to drive, consider avoiding alcohol or installing an ignition interlock device that prevents your car from starting without a breath sample.
Why Test?
Alcohol intoxication is legally determined by the blood alcohol concentration (BAC) level. However, collecting a blood sample at the scene for lab analysis is neither practical nor efficient for detaining drivers suspected of driving while impaired (DWI) or driving under the influence (DUI). Similarly, urine tests for alcohol are as impractical as blood sampling in the field. What was needed was a method to measure something related to BAC without invasive procedures on the suspect's body.
In the 1940s, law enforcement first began developing breath alcohol testing devices. In 1954, Dr. Robert Borkenstein from the Indiana State Police invented the Breathalyzer, a type of breath alcohol testing device still widely used by police forces today.
Now, let’s delve into the science behind these tests.
Detecting Alcohol: the Principle of Testing
Alcohol from a drink appears in the breath because it is absorbed into the bloodstream through the mouth, throat, stomach, and intestines.
Alcohol is not digested or chemically altered once absorbed; it remains unchanged in the bloodstream. As blood flows through the lungs, some alcohol passes from the blood across the lung's air sacs (alveoli) into the air because alcohol is volatile — it evaporates from a solution. The concentration of alcohol in the alveolar air is directly linked to the concentration in the blood.
As the alcohol in the alveolar air is exhaled, it can be detected by breath alcohol testing devices. Instead of needing to draw blood, officers can test a driver's breath immediately and determine if there’s enough evidence to make an arrest. Breathalyzers work so efficiently because they understand how alcohol is processed by the body.
How Much Alcohol Is Too Much?
Since alcohol concentration in the breath correlates with that in the blood, BAC can be estimated by measuring alcohol in the breath. The ratio between breath alcohol concentration and blood alcohol content is 2,100:1. This means 2,100 milliliters (ml) of alveolar air contain the same alcohol amount as 1 ml of blood.
For many years, the legal intoxication standard in the U.S. was set at a BAC of 0.10, though most states have since lowered it to 0.08. The federal government has encouraged states to adopt the lower limit. According to the American Medical Association, impairment can occur when BAC reaches 0.05. A BAC of 0.08 means 0.08 grams of alcohol per 100 ml of blood.
Different Breathalyzer Technologies
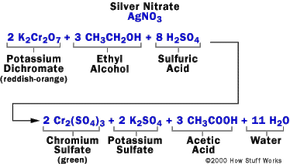 Illustration showing the chemical process in a breathalyzer that detects alcohol.
Illustration showing the chemical process in a breathalyzer that detects alcohol.Breath alcohol testing devices come in three primary types, each relying on distinct scientific principles:
- Breathalyzer - Utilizes a chemical reaction with alcohol that causes a visible color change
- Intoxilyzer - Identifies alcohol through infrared (IR) spectroscopy
- Alcosensor III or IV - Detects alcohol through a chemical reaction within a fuel cell
Regardless of the model, each breathalyzer includes a mouthpiece, a tube for the suspect to blow into, and a sample chamber where the air is collected. The internal components differ based on the device type.
How Breathalyzers Operate
The Breathalyzer consists of the following components:
- A mechanism to collect the suspect's breath sample
- Two glass vials containing the chemical reaction solution
- A set of photocells linked to a meter that tracks the color change caused by the chemical reaction
To detect alcohol molecules, the suspect exhales into the device. The breath is then passed through a mixture of sulfuric acid, potassium dichromate, silver nitrate, and water in one vial.
Chemical Reactions
The measurement process relies on the following chemical reaction:
- The sulfuric acid extracts the alcohol from the air, turning it into a liquid form.
- The alcohol interacts with potassium dichromate, resulting in: chromium sulfate, potassium sulfate, acetic acid, and water.
Silver nitrate acts as a catalyst, speeding up the reaction without being consumed by it. The sulfuric acid not only removes the alcohol but also potentially creates the acidic environment necessary for the reaction to occur.
As the reaction takes place, the reddish-orange dichromate ion turns green upon reacting with the alcohol. The extent of this color change is directly linked to the alcohol content in the exhaled air. To measure this, the reacted solution is compared with an unreacted sample in the photocell system, which generates an electric current that moves the meter needle from its resting position.
The operator then adjusts a knob to return the needle to its original position and reads the alcohol level from the knob. The more the knob needs to be turned, the higher the alcohol level present in the sample.
The alcohol in alcoholic drinks is ethyl alcohol (ethanol). Its molecular structure is represented as follows:
where C stands for carbon, H is hydrogen, O is oxygen, and each hyphen represents a bond between atoms. The bonds between the three hydrogen atoms and the carbon atom on the left are omitted for clarity.
The OH (O - H) group is what categorizes the molecule as an alcohol. This molecule contains four types of bonds:
- carbon-carbon (C - C)
- carbon-hydrogen (C - H)
- carbon-oxygen (C - O)
- oxygen-hydrogen (O - H)
The chemical bonds between atoms involve pairs of shared electrons. Much like springs, these bonds can bend and stretch, which plays an important role in detecting ethanol using infrared (IR) spectroscopy.
Devices Overview: Intoxilyzer
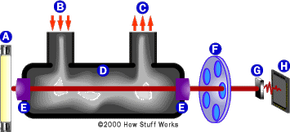 Diagram of the Intoxilyzer device in detail.
Diagram of the Intoxilyzer device in detail.This device utilizes infrared (IR) spectroscopy, a technique that identifies molecules by the specific way they absorb IR light.
Molecules are in constant motion, vibrating, and these vibrations alter when they absorb IR light. These alterations include the bending and stretching of different chemical bonds. Each type of bond absorbs IR light at distinct wavelengths.
To identify ethanol in a sample, the focus is on the wavelengths of the bonds in ethanol (C-O, O-H, C-H, C-C), and the absorption of IR light is measured. The absorption patterns help confirm the presence of ethanol, while the amount of absorption indicates how much ethanol is present.
 Detailed diagram of the Intoxilyzer device.
Detailed diagram of the Intoxilyzer device.In the Intoxilyzer device:
- A lamp produces a broad-spectrum (multiple-wavelength) infrared (IR) beam.
- The IR beam passes through the sample chamber and is focused by a lens onto a rotating filter wheel.
- The filter wheel is equipped with narrow-band filters designed for the wavelengths specific to the bonds in ethanol. The light that passes through each filter is detected by a photocell, which then converts it into an electrical pulse.
- The electrical pulse is sent to the microprocessor, which processes the pulses and calculates the BAC by analyzing the absorption of infrared light.
How the Alcosensor III or IV Works
Modern fuel-cell technology, which might someday power our vehicles and even homes, has been integrated into breath-alcohol detectors. Devices such as the Alcosensor III and IV operate using fuel cell sensors.
The fuel cell consists of two platinum electrodes separated by a porous acid-electrolyte material. When the suspect exhales air over one side of the fuel cell, the platinum electrodes oxidize any alcohol present, producing acetic acid, protons, and electrons.
The electrons flow through a wire from one platinum electrode to the other. The wire is connected to an electrical current meter, and the platinum electrode on the opposite side. Protons travel through the lower section of the fuel cell, where they combine with oxygen and electrons to form water. The amount of alcohol oxidized determines the magnitude of the electrical current. A microprocessor measures this current and calculates the BAC.
Breath Tests in a Legal Setting
Operators of breath alcohol testing equipment must undergo proper training to operate and calibrate the device, particularly when the results are intended as evidence in DWI cases. Law enforcement officers may also use portable breath testing devices that work based on the same principles as full-sized models.
Court cases often depend on the perceived reliability of breath test results, which is why prosecutors prefer using data from full-sized devices. So, if you’re drinking, avoid the risk of jail time or other consequences by choosing not to drive.
For more details on Breathalyzers and related topics, explore the links provided below.
When hydrogens are removed from the right carbon of ethanol in the presence of oxygen, acetic acid is formed, which is the primary ingredient in vinegar. The molecular structure of acetic acid is represented as:
Here, C stands for carbon, H for hydrogen, O for oxygen, with a hyphen indicating a single chemical bond between atoms and a || symbol indicating a double bond. For simplicity, the three hydrogen atoms attached to the left carbon atom are omitted. As ethanol undergoes oxidation to become acetic acid, two protons and two electrons are also released.
