 Flying in a hot air balloon promises some truly awe-inspiring vistas.
CargoLifter
Flying in a hot air balloon promises some truly awe-inspiring vistas.
CargoLifterHot air balloons are far from practical for traveling long distances—they can't be steered, and they only move with the wind. But for those seeking the joy of flight, nothing compares to it. Many describe ballooning as one of the most tranquil, enjoyable activities they've ever experienced.
Hot air balloons are a clever application of fundamental scientific concepts. Ever wondered how hot air balloons work? In this article, we'll discover what makes these balloons rise into the sky, and how the design allows the pilot to control altitude and vertical speed. You'll be amazed at the elegant simplicity of these early flying machines.
Hot air balloons operate on a fundamental scientific principle: warm air rises in cooler surroundings. Simply put, hot air is lighter than cool air due to having less mass per unit of volume. A cubic foot of air weighs around 28 grams (about an ounce). If this air is heated by 100°F, it becomes roughly 7 grams lighter. Consequently, each cubic foot of air in a hot air balloon can lift approximately 7 grams. This is why hot air balloons are so large — to lift 1,000 pounds, you'd need about 65,000 cubic feet of hot air.
Next, we’ll examine the various parts of hot air balloons to understand how they heat the air.
Rising Balloons
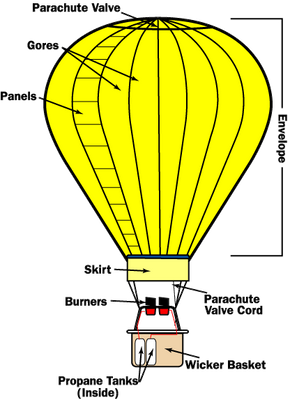 A hot air balloon consists of three key components: the burner, which heats the air; the envelope, which holds the air; and the basket, which carries the passengers.
Mytour
A hot air balloon consists of three key components: the burner, which heats the air; the envelope, which holds the air; and the basket, which carries the passengers.
MytourTo keep the balloon in the air, reheating the air is essential. Hot air balloons achieve this by using a burner located beneath an open balloon envelope. As the air inside the balloon cools down, the pilot can reignite it by activating the burner.
Modern hot air balloons use propane, the same fuel often used in outdoor grills, to heat the air. The propane is stored in lightweight cylinders as a compressed liquid, placed within the balloon's basket. A hose draws the liquid out from the bottom of the cylinder.
Due to the high compression of the propane in the cylinders, it flows rapidly through the hoses toward the heating coil. This coil is simply a steel tube bent into a spiral around the burner. When the burner is ignited, propane in liquid form is released and lit by a pilot light.
As the flame ignites, it heats the metal tubing surrounding it. This causes the tubing to warm up, transferring heat to the propane flowing inside. This process converts the propane from liquid to gas, which is then ignited. The gas results in a stronger flame and more efficient fuel use.
Most modern hot air balloons are made with long nylon gores, which are reinforced with sewn-in webbing. These gores stretch from the base of the envelope to its crown and are formed from smaller panels. Nylon is ideal for balloons because it's light, durable, and has a high melting point. The skirt of the envelope, which is the nylon at the base, is coated with a fire-resistant material to prevent the flame from igniting the balloon.
 The basket is responsible for carrying the passengers, propane tanks, and navigation instruments.
The basket is responsible for carrying the passengers, propane tanks, and navigation instruments.The hot air remains trapped inside the envelope due to buoyancy, which causes it to rise. If the pilot keeps the fuel jets firing, the balloon will continue to ascend. However, there is a maximum altitude, as the air becomes too thin for buoyancy to maintain lift. The buoyant force is determined by the weight of the air displaced by the balloon, so a larger envelope can typically reach a higher altitude than a smaller one.
Wicker is the most commonly used material for the passenger compartment of hot air balloons. It is strong, flexible, and light. This flexibility is beneficial during landings, as it absorbs some of the impact force. Passengers in a more rigid basket would feel the full force of landing.
Piloting a Balloon
 To ignite the burner, the pilot opens the propane valve.
CargoLifter
To ignite the burner, the pilot opens the propane valve.
CargoLifterFlying a balloon requires skill, though the controls are quite straightforward. To make the balloon rise, the pilot adjusts a control that opens the propane valve. This lever functions similarly to the knobs on a gas grill or stove: turning it increases the gas flow, which makes the flame grow larger. The pilot can adjust the vertical speed by creating a bigger flame to heat the air more quickly.
Moreover, many hot air balloons feature a control that opens a second propane valve. This valve directs propane through a hose that bypasses the heating coils. It allows the pilot to burn liquid propane rather than propane in gas form. While burning liquid propane produces a weaker, less efficient flame, it is much quieter compared to burning gas. Pilots often use this second valve when flying over livestock farms to avoid startling the animals.
 The parachute valve is located inside the balloon. A Kevlar cord extends from the valve at the top of the balloon, down to the basket, and through the center of the envelope.
CargoLifter
The parachute valve is located inside the balloon. A Kevlar cord extends from the valve at the top of the balloon, down to the basket, and through the center of the envelope.
CargoLifterHot air balloons also have a cord that opens the parachute valve at the top of the envelope. When the pilot pulls the cord, hot air escapes from the envelope, which reduces the inner air temperature and slows the balloon’s ascent. If the valve remains open for long enough, the balloon will begin to descend.
These are essentially the only controls — heat for rising and venting for sinking. This leads to an intriguing question: If pilots can only move the balloon up and down, how do they navigate it from one location to another? The answer lies in the fact that wind currents vary with altitude. To move in a specific direction, a pilot changes their vertical position, ascends or descends to the right altitude, and lets the wind carry them. Wind speeds generally increase with altitude, which also allows pilots to adjust their horizontal speed by altering their altitude.
However, even the most experienced pilots don’t have full control over the balloon's flight path. Wind conditions typically limit the pilot’s options. As a result, it is nearly impossible to pilot a hot air balloon along a precise course. It’s also very uncommon for a pilot to be able to bring the balloon back to its original starting point.
Unlike flying an airplane, piloting a hot air balloon is largely an improvised process, dependent on the moment. Because of this, some members of the balloon crew must stay on the ground, following the balloon in a car to track its landing. Once it lands, they can retrieve the passengers and equipment.
Launching and Landing
 Getting ready to safely launch a hot air balloon requires significant effort.
Getting ready to safely launch a hot air balloon requires significant effort.Much of the work in hot air ballooning happens before takeoff and after landing, when the crew inflates and deflates the balloon. For the audience, this is often more exciting to watch than the flight itself.
After selecting an appropriate launch site, the crew attaches the burner system to the basket. Next, they connect the balloon envelope and begin spreading it out on the ground.
After the envelope is spread out, the crew begins to inflate it using a large, powerful fan positioned at the base of the envelope.
 CargoLifter
CargoLifterOnce enough air fills the balloon, the crew directs the burner flame into the opening of the envelope. This heats the air, increasing the pressure until the balloon inflates fully and begins to rise off the ground.
 CargoLifter
CargoLifterThe ground crew secures the balloon basket, keeping it in place until the launch team is fully on board. It is also fastened to the ground vehicle right until the last moment, preventing the balloon from drifting away before the flight is ready. Once everything is in position, the ground crew releases the balloon and the pilot ignites a consistent flame from the burner. As the air inside warms up, the balloon ascends smoothly from the ground.
Incredibly, this entire operation takes just 10 to 15 minutes. The process of landing, deflating, and packing up the balloon envelope takes a bit more time.
Before landing, the pilot discusses possible landing zones with the ground team using an onboard radio. They aim for an open area, free from power lines, and large enough to safely unload the balloon. The pilot is always on the lookout for possible landing sites once the balloon is airborne, in case of an emergency.
Landing the balloon can sometimes be bumpy, but an experienced pilot will guide it gently to the ground, minimizing any hard impact. If the ground team has reached the site, they will hold the basket in place once the balloon touches down. If the landing position is not ideal, the crew will move the balloon to a better location.
The ground crew lays down a protective tarp to shield the balloon from damage. The pilot then fully opens the parachute valve, allowing the air to escape from the top of the balloon. The ground team pulls a cord attached to the balloon's top and drags the envelope onto the tarp.
After the balloon envelope lands on the ground, the crew begins releasing the air. Once the balloon is fully deflated, they fold it up and pack it into a stuff sack. This process is similar to packing a giant sleeping bag.
We extend our gratitude to CargoLifter for their assistance with this article.
Wind and Weather
 The pilot releases a helium-filled piball to determine the wind direction.
The pilot releases a helium-filled piball to determine the wind direction.Before taking off, pilots consult a weather service to gather information about the climate and wind conditions in the area. Cautious pilots only fly when conditions are nearly perfect — clear skies and moderate winds.
Storms pose a significant threat to hot air balloons due to the risk of lightning strikes. Even rain can be problematic, as it reduces visibility and damages the balloon's material (not to mention that flying in wet weather is generally unpleasant). While some wind is necessary for a smooth flight, strong winds could easily destroy the balloon.
Pilots also call the weather service to get an idea of the balloon's potential flight path and how to navigate once in the air. In addition, a pilot may release a piball (a helium-filled balloon) to assess the wind's exact direction at a potential launch site. If the wind appears to steer the balloon into restricted airspace, the crew must choose a different launch site.
 The pilot carries various instruments onboard the balloon.
The pilot carries various instruments onboard the balloon.In flight, the pilot uses an onboard altimeter, variometer, and their own judgment to find the ideal altitude. Achieving the correct altitude is challenging because there's at least a 30-second delay between firing the burners and the balloon actually lifting off.
Balloon pilots must operate the necessary controls slightly ahead of when they want to ascend and stop them just before they want to level off. Inexperienced pilots often overcompensate, rising too high before stabilizing. Mastery of these controls comes only after many hours of flying.
Now that we've seen how a hot air balloon soars through the sky, let's explore the forces that make this possible. Hot air balloons are a remarkable showcase of some of the most fundamental forces on earth.
Air: A High-Pressure Fluid
One of the fascinating aspects of life on Earth is that we are constantly immersed in a high-pressure fluid — a substance that has mass but no fixed shape. The air surrounding us is made up of various elements in a gaseous form. In this gas, the atoms and molecules of the elements move freely, colliding with one another and everything else.
As these particles strike an object, each transfers a small amount of energy. Because there are so many particles in the air, the cumulative effect of their collisions results in significant pressure. At sea level, this pressure is about 14.7 pounds per square inch (psi) or 1 kg per square centimeter (kg/cm²).
The force of air pressure relies on two factors:
- The frequency of particle collisions — the more collisions that occur in a given time frame, the greater the energy transferred to an object.
- The intensity of the impact — if the particles collide with more force, they transfer more energy to an object.
These factors are influenced by the number of air particles in a specific area and their speed. More particles or faster-moving particles result in more frequent collisions, leading to higher pressure. Additionally, increasing the speed of the particles also amplifies the force of their impacts.
We often don't notice air pressure because it's all around us. In a space where conditions are uniform, air particles spread out evenly, ensuring that the air density is the same at every point. If no other forces are present, this results in consistent air pressure everywhere. We don't feel the effect of this pressure because the forces from all directions cancel each other out.
For instance, 14.7 psi is enough to knock over or crush a chair from above. However, because the air exerts nearly the same pressure from every direction — right, left, top, bottom, and others — the forces on the chair balance each other out. Therefore, the chair doesn't experience significantly more pressure from any one direction.
In a world without other influences, air pressure would be perfectly balanced, with equal force from all directions. But on Earth, we must account for additional forces, especially gravity.
Although air particles are very tiny, they do have mass, which causes them to be pulled toward the Earth. This pull is minimal at any given level of the atmosphere — the air particles appear to move in straight lines without noticeably falling toward the ground. Thus, the pressure is mostly balanced on a small scale. However, gravity does exert a pull that gradually increases the pressure as we approach the Earth's surface.
In the following section, we will delve into the mechanics of how this process works.
Air Pressure + Gravity = Buoyancy
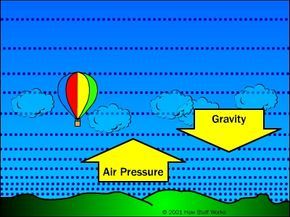 Gravity and air pressure combine to create the buoyant force needed for a hot air balloon.
Gravity and air pressure combine to create the buoyant force needed for a hot air balloon.Air particles in the atmosphere are all pulled downward by gravity. However, the air pressure pushes upward, counteracting gravity. The air density increases to the level where it balances gravity's force, as gravity cannot pull down more particles beyond this point.
The air pressure is strongest right at the Earth's surface because it is supporting the weight of the air above it. The greater the weight above, the stronger the downward pull of gravity. As you ascend through the atmosphere, the weight of the air above lessens, and the balancing pressure diminishes, which explains why pressure decreases with altitude.
This variation in air pressure creates an upward buoyant force throughout the surrounding air. Essentially, air pressure below objects is higher than it is above them, causing air to push upwards more than it pushes down. However, this buoyant force is much weaker than gravity's pull — it is only as strong as the weight of the air displaced by an object. Clearly, most solid objects are heavier than the air they displace, so buoyant force doesn't have much effect. The buoyant force can only affect things that are lighter than the air around them.
In order for buoyancy to lift an object, that object must be lighter than the surrounding air. The most obvious thing lighter than air is a vacuum. A vacuum can occupy space but has no mass, so one might think that a balloon filled with a vacuum should be buoyed up by the surrounding air. However, this doesn't happen due to the effect of external air pressure.
Air pressure does not crush an inflated balloon because the air inside the balloon exerts an outward force equal to the inward force of the outside air. However, a vacuum doesn't exert any outward force, as there are no particles to collide with anything. Without this balance of forces, the external air pressure easily crushes the balloon. Additionally, any container strong enough to withstand the air pressure at the Earth's surface would be far too heavy to be lifted by buoyant force.
Another possibility is to fill the balloon with air that is less dense than the surrounding air. Since the air inside the balloon would have less mass per unit of volume than the atmospheric air, it would be lighter, allowing the buoyant force to lift the balloon. However, fewer air particles per unit means lower air pressure, and the surrounding air pressure would compress the balloon until the air inside reaches the same density as the outside air.
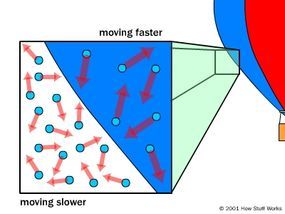 Inside the balloon, there are fewer air particles per unit of volume, but because these particles move more quickly, the pressure inside and outside the balloon remains equal.
Inside the balloon, there are fewer air particles per unit of volume, but because these particles move more quickly, the pressure inside and outside the balloon remains equal.This assumes that the air inside the balloon and the air outside it are under the exact same conditions. If we alter the conditions of the air within the balloon, we can decrease its density while maintaining the same air pressure. As we saw earlier, the pressure exerted by air on an object depends on the frequency of collisions with that object, as well as the force of each collision. We can increase the overall pressure in two ways:
- Increase the number of air particles, resulting in more frequent particle impacts on a given surface area.
- Increase the velocity of the particles, so they collide more often and with more force.
To reduce the air density in a balloon without reducing air pressure, all that is needed is to increase the speed of the air particles. This can be easily achieved by heating the air. As the air particles absorb the heat energy, they become more energetic, causing them to move faster. This leads to more frequent collisions with a surface and greater force with each collision.
Hot air exerts greater pressure per particle than cold air, which means fewer particles are needed to reach the same pressure level. This is why a hot air balloon rises: it contains hot, less dense air, while being surrounded by cooler, denser air.
History of Ballooning
The concept behind hot air balloons has been around for centuries. Over 2,000 years ago, the brilliant Greek mathematician Archimedes discovered the principle of buoyancy and may have even imagined flying machines that could be lifted by this force. In the 13th century, English scientist Roger Bacon and German philosopher Albertus Magnus both suggested the idea of flying machines based on buoyancy.
However, it wasn't until the summer of 1783 that flight truly began to take shape. That year, the Montgolfier brothers launched a sheep, a duck, and a chicken on an eight-minute flight in France. Joseph and Etienne Montgolfier, who worked for their family's paper company, were experimenting with heated air to lift paper vessels into the sky.
Over the next few years, they refined their design, creating a hot air balloon resembling the ones we use today. Instead of propane, however, they fueled their creation with a fire pit that burned straw, manure, and other materials.
On September 19, 1783, the Montgolfiers conducted their first demonstration flight for King Louis XVI, with the sheep, duck, and chicken as passengers. The animals survived the flight, reassuring the King that humans could likely breathe at such altitudes. Two months later, Marquis François d'Arlandes, an infantry major, and Pilâtre de Rozier, a physics professor, became the first humans to soar in a hot air balloon.
Other designs for hot air balloons and daring flights emerged, but by 1800, gas balloons had largely taken over. One significant reason for the decline of hot air balloon popularity was the tragic death of Pilâtre de Rozier during a flight attempt over the English Channel. The balloon he used for the flight featured both a smaller hydrogen balloon and the hot air balloon envelope. Unfortunately, the hydrogen caught fire early in the flight, causing the entire balloon to explode.
The primary reason hot air balloons fell out of favor, however, was the superior capabilities of gas balloon dirigibles, which boasted advantages such as longer flight durations and the ability to be steered.
Another type of balloon that gained popularity was the smoke balloon. These balloons were powered by a fire on the ground, without any attached heat source. They would shoot upwards and then descend back to the ground.
Their main function was as a spectacle at traveling fairs in the United States during the late 1800s and early 1900s. The balloonist would wear a parachute and attach themselves to a canvas balloon. Several assistants would hold the balloon above a fire pit, heating the air to increase its upward force. Once the force was sufficient — and the balloon had avoided catching fire — the assistants would release it, sending the balloonist into the sky. At the peak of the flight, the balloonist would detach and parachute back to the ground.
Since the 1960s, traditional hot air balloons have experienced a revival, thanks in part to Ed Yost and his company, Raven Industries. In 1956, Yost and his partners founded Raven Industries to design and construct hot air balloons for the United States Navy's Office of Naval Research (ONR), which needed them for short-range transport of small loads. Yost and his team took the basic Montgolfier balloon concept and enhanced it, adding a propane burner system, new envelope material, a new inflation method, and a range of critical safety features.
They also invented the modern, lightbulb-shaped envelope. Initially, Yost created large, spherical balloons. These worked well but had a peculiar inflation pattern: when the air was heated, the top of the balloon inflated, but the bottom remained under-inflated. To make them more efficient, Yost removed the extra fabric from the bottom, resulting in the familiar 'natural' balloon shape we know today.
By the early 1960s, the ONR had lost interest in hot air balloons, prompting Yost to begin selling his creations as sporting equipment. As interest in ballooning grew, new companies emerged. Over time, designers continued to refine hot air balloons, introducing new materials, safety features, and creative envelope shapes. Some manufacturers even increased the size of the baskets and load capacities, with some balloons now able to carry up to 20 passengers!
Despite all the changes, the fundamental design still follows Yost’s modification of the Montgolfier brothers' original concept. This groundbreaking technology has captivated people worldwide. Balloon tours have become a multi-million dollar industry, and balloon races and other events continue to draw large crowds. It has even become trendy among billionaires to create advanced high-tech balloons for globe-spanning adventures. The enduring popularity of hot air balloons, even in the era of jet planes, helicopters, and space shuttles, speaks volumes about their lasting appeal.
For further exploration into the world of hot air balloons and related subjects, check out the following links.
What’s it like to soar in a hot air balloon? It’s an incredibly peaceful and tranquil experience. Since the balloon drifts with the wind, you won’t feel any breeze. Without the strong winds typically associated with high altitudes, flying feels safe and soothing. You simply lift off the ground and glide with the air in the atmosphere.
