 Aerogels are often referred to as 'frozen smoke' due to their ethereal, bluish hue.
NASA/JPL-Caltech
Aerogels are often referred to as 'frozen smoke' due to their ethereal, bluish hue.
NASA/JPL-CaltechAerogel, a substance that emerged from a wager between two scientists in the late 1920s, could very well be the most extraordinary material on Earth. It's the lightest solid known to man — even earning a spot in the Guinness World Records — and yet, it has the ability to hold 500 to 4,000 times its own weight [source: NASA JPL, Guinness; Steiner, Zero-Gravity]. A single cubic inch of aerogel could cover an entire football field. It's not only breathable and fire-resistant, but it can also absorb both oil and water.
Despite its lightness, aerogel is surprisingly durable. While aerogel insulation can be an excellent electrical conductor, it can also serve as an efficient thermal insulator when made from various materials [source: Steiner, Zero-Gravity].
In this article, we'll delve into the uniqueness of aerogels, from their discovery in California in the late 1920s to their mission in 1999 to capture space dust. We'll also look ahead to the future of aerogels, exploring whether they can be made more affordable for the average person. Plus, we'll show you how to create your own aerogel — believe it or not, it's possible!
Why Aren't Aerogel Particles More Well-Known?
After learning about aerogel's rare and remarkable properties, you might be asking: Why isn't aerogel insulation more widely recognized? The answer lies in the immense time and cost required to produce such an extraordinary material, as only small quantities are made in each production run.
Although increasing aerogel production would reduce its cost, the materials and methods involved still come at a steep price of about $1.00 per cubic centimeter. At approximately $23,000 per pound, aerogel currently costs more than gold [source: NASA JPL, FAQs]!
Such a precious substance might seem more fitting in the jewelry collection of an heiress, right next to diamonds and pearls. But in reality, aerogel is more likely to be found insulating spacecraft or enhancing paints than dazzling the wealthy elite. Though aerogels may lack the shine of gold, their performance is unmatched.
Keep reading to discover how the lightest solid on Earth first made its debut and how this incredibly versatile material is created.
The Story Behind Aerogel
The origins of aerogel are steeped in mystery, but we do know that in the late 1920s, American chemist Samuel Kistler made a bet with his colleague Charles Learned. Kistler theorized that what made an object a gel was not its liquid content but its structure, specifically the nanopores — tiny, microscopic openings within the material. When he attempted to demonstrate this by simply evaporating the liquid, the gel collapsed like a soufflé. The goal was to be the first to replace the liquid in a gel with gas without damaging its structure [source: Steiner, Zero Gravity].
After numerous experiments, Kistler succeeded in replacing the liquid in the gel with gas, creating a substance that was structurally still a gel but without any liquid. In 1931, he published his breakthrough in the scientific journal *Nature*, in an article titled 'Coherent Expanded Aerogels and Jellies' [source: Ayers, Pioneer].
Aerogel starts as a gel known as alcogel. Alcogel is a silica gel, amorphous in nature, with alcohol trapped in its nanopores. If the alcohol were simply evaporated, the structure would collapse, much like a wet sponge drying on a countertop. Instead of just relying on evaporation, the gel undergoes a process known as supercritical drying. Here’s how it’s done:
- Pressurize and heat the gel past its critical point, where gas and liquid properties converge at high temperatures.
- Depressurize the gel while maintaining its high temperature. As the pressure drops, the alcohol vaporizes and the gel becomes less dense.
- Remove the gel from the heat source. As it cools, there’s too little alcohol remaining to recondense into liquid, so it remains as gas.
- Inspect your final product. You’ll now have a silica-based solid, filled with gas (air) where liquid once was.
Supercritical drying is the process through which the liquid "alco" in alcogel transforms into a gas within the nanopores of silica, without causing the structure to collapse. Once the alcohol is removed, the alcogel becomes aerogel, a material where air takes the place of alcohol. Aerogel is a highly porous substance, maintaining only 50 to 99 percent of the original material's volume, making it lightweight, flexible, and versatile [source: Steiner, Zero Gravity].
Proceed to the following page to discover the various types of aerogels commonly utilized today.
Types of Aerogels
The three most widely used types of aerogels are silica, carbon, and metal oxides. However, silica is the most prevalent, both in research and practical applications. When people refer to aerogels, they are most often referring to the silica variety [source: Aerogel.org, Silica
Carbon Aerogel Insulation Material
In contrast to the smoky-blue appearance of silica aerogels, carbon-based aerogels are black and have a texture similar to charcoal. While they may not be as visually striking, their remarkable properties, such as a high surface area and the ability to conduct electricity, make them valuable for applications like supercapacitors, fuel cells, and desalination systems [source: Aerogel.org, Organic].
Metal Aerogel Insulation Material
Metal oxide aerogels, derived from metal oxides, serve as catalysts in chemical reactions. These aerogels also play a role in producing explosives and carbon nanotubes, and some even exhibit magnetic properties. What makes metal oxide aerogels, like iron oxide and chromia, stand out from their silica counterparts is their vibrant colors. When turned into aerogels, iron oxide gives off a distinctive rust hue, while chromia aerogels appear in deep shades of green or blue. Each metal oxide yields a different color when transformed into an aerogel [source: Aerogel.org, Metal].
Silica Aerogel Insulation Material
Silica aerogels, the most common type, are blue for the same reason the sky is blue. This blue color emerges when white light interacts with the aerogel's silica molecules, which are larger than the wavelengths of light. The aerogel scatters shorter wavelengths, like blue and violet, more effectively than longer wavelengths, with blue light being the most scattered. Our eyes are more sensitive to blue, so we perceive the blue wavelengths and not the violet ones [source: Steiner, Zero-Gravity].
Discover the fascinating uses of aerogels in space exploration.
Alcogels contain alcohol in their pores, but what would happen if water was used instead? In his early experiments, Kistler worked with hydrogels, which are filled with water. When dried, these gels act similarly to Jell-O: they collapse into a sticky, messy substance because the water evaporates too quickly for the structure to maintain its form. As molecules escape, others rush in to fill the gaps, creating what's called capillary stress within the gel's pores, causing the entire structure to disintegrate [source: Hunt and Ayers, History].
Aerogels in Space
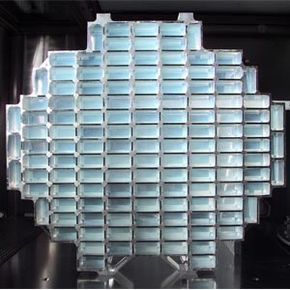 This dust collector for the STARDUST spacecraft was equipped with 260 panels of aerogel.
NASA/JPL-Caltech
This dust collector for the STARDUST spacecraft was equipped with 260 panels of aerogel.
NASA/JPL-CaltechAerogel's adaptability has made it an essential material both on Earth and in space. It has served many functions in various NASA missions, including insulating the electrical systems of the Mars rovers with aerogel blankets and capturing space dust from a rapidly moving comet.
In 1999, NASA embarked on a mission where a spacecraft traveled 4.8 billion kilometers (roughly 6,000 trips to the moon) to reach comet Wild 2. Upon arrival, the dust collector, shaped like a tennis racket, deployed its 260 aerogel cubes to capture fast-moving interstellar particles and preserve them in their natural state [source: NASA JPL, Aerogel].
As particles collided with the dust collector, they left trails inside the aerogel cubes as they slowed down. These trails helped scientists easily locate the tiny space particles. The aerogel's resilience ensured that the dust collector returned intact, with all aerogel tiles still in place. Researchers are now studying the dust and crystals trapped in the aerogel, eagerly awaiting the knowledge they may provide [source: Bridges].
Now, let's explore some of the commercial applications of aerogel.
Everyday Aerogel Uses
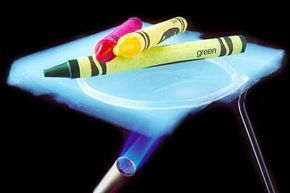 The aerogel protects the crayons above it from the flame beneath. Similar silica aerogels have been used to insulate the Mars rover.
NASA/JPL-Caltech
The aerogel protects the crayons above it from the flame beneath. Similar silica aerogels have been used to insulate the Mars rover.
NASA/JPL-CaltechIn the early years, aerogels were marketed as thickening agents and found their way into various products such as makeup, paint, and even napalm. They were also used in cigarette filters and freezer insulation. Monsanto was the first to commercialize aerogel's uses. However, Kistler's supercritical drying process, although effective, was also hazardous, labor-intensive, and costly. After three decades of production, these challenges led Monsanto to discontinue its focus on aerogels in the 1970s.
Yet, this was not the end for aerogel's potential as thermal insulation. Soon after Monsanto halted production, scientists discovered a new process that made aerogel production safer. By substituting a less toxic alkoxide compound and using supercritical carbon dioxide instead of alcohol in the drying phase, they made the process less dangerous and time-consuming. These improvements reduced both the hazardous nature of production and the drying time, making aerogels more commercially viable. As a result, interest in the material grew [source: Hunt and Ayers, History].
With production becoming safer and more efficient, aerogel's exceptional properties, including its thermal conductivity, have made it valuable across a variety of industries. Silicon manufacturers, builders of home materials, and space agencies have all utilized aerogel. Its cost has been the main barrier to widespread use, though there is a growing effort to create more affordable versions. In the meantime, aerogels can be found in a wide array of products:
- Wetsuits
- Firefighter suits
- Skylights
- Windows
- Rockets
- Paints
- Cosmetics
- Nuclear weapons
[source: Aerogel.org, Modern History]
A Marvel in Thermal Management
Thanks to its remarkable structure, aerogel is an ideal insulator. The air pockets within aerogel’s structure effectively counteract all three forms of heat transfer: convection, conduction, and radiation [source: Cabot Corporation]. While aerogel remains costly, studies show that using it for insulation in wall framing and other challenging areas, such as window flashing, can save homeowners up to $750 annually.
Not only does aerogel insulation help homeowners save money, but it also reduces carbon footprints significantly [source: Aspen Aerogels, New Spaceloft]. Companies are racing to reduce costs, but for now, aerogels are more accessible to NASA than to the general public. However, construction companies, power plants, and refineries are already making use of aerogels. With further cost reductions, aerogel could soon become the breakthrough material it deserves to be.
From Earth to space, aerogels undeniably have a promising future. Continue reading to explore the latest advancements in aerogel technology and how you can start experimenting with aerogel yourself.
The Future of Aerogels
 A 5.5-pound brick is held up by a mere 2-gram (0.07 ounces) piece of silica aerogel.
NASA/JPL-Caltech
A 5.5-pound brick is held up by a mere 2-gram (0.07 ounces) piece of silica aerogel.
NASA/JPL-CaltechAlthough aerogel is costly, researchers continue to explore ways to make it stronger, more affordable, and safer. In 2002, Professor Nicholas Leventis from the Missouri University of Science and Technology stunned the scientific community by unveiling a method for creating non-brittle aerogels.
Leventis's innovation, known as x-aerogels, not only makes the material stronger but also more flexible, waterproof, and impact-resistant. However, the production of x-aerogels requires more hazardous chemicals and extended production times, which can reduce their insulating properties [source: Aerogel.org, Strong]. Despite these drawbacks, x-aerogels could be applied in the following areas:
- Insulating skylights
- Armor
- Non-deflatable (or "run-flat") tires
- Membranes for electrochemical cells
- Aircraft structural components
- Heat shields for spacecraft reentry
[source: Leventis]
Aerogels could play a pivotal role in advancing green technology. Specifically, carbon aerogel shows immense promise in powering energy-efficient vehicles through its use in supercapacitors and fuel cells. Its impressive energy storage capacity could give rise to a host of new technologies, but this depends on making aerogel production more cost-effective for large-scale manufacturing.
Interested in Creating Your Own?
The great news is that you don't need a research grant to start experimenting with aerogel creation. While it is possible to create aerogels at home, it's recommended to do so in a well-equipped laboratory that provides all the necessary materials, including an autoclave for supercritically drying the aerogel. (Feeling industrious? Here are instructions on building your own supercritical dryer.) Reach out to local universities or community colleges, and they may allow you to use their equipment if you have a recipe you want to try [source: Hunt and Ayers, Making; Aerogel.org, Build].
Many websites, including aerogel.org, offer detailed guides on how to make your own aerogels. No matter where you choose to make your aerogel, always prioritize safety. Wear goggles, gloves (dishwashing gloves work well), long pants, closed-toe shoes, and a painter's mask to protect yourself from toxic fumes and flammable materials [source: Steiner, How to Make; Hunt and Ayers, Making].
