 Fluoroantimonic acid holds the title of the most potent superacid discovered. Wikimedia/(CC BY-SA 3.0)
Fluoroantimonic acid holds the title of the most potent superacid discovered. Wikimedia/(CC BY-SA 3.0)Certain acids, such as muriatic acid, are safe for household use, provided safety guidelines are followed. However, others are far too dangerous for casual handling due to their highly corrosive nature.
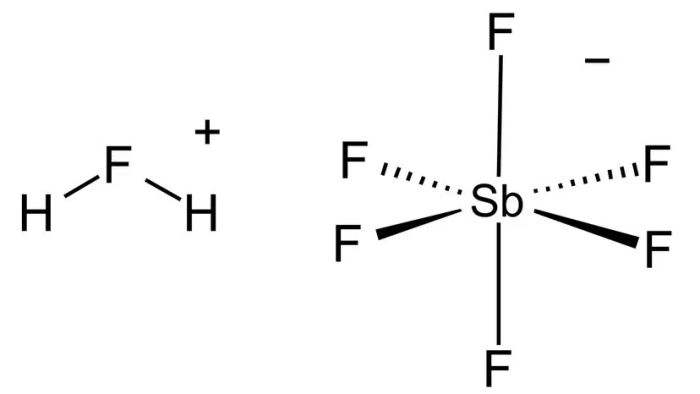
What occurs when two such powerful acids are combined? The outcome is extraordinary. For example, mixing hydrogen fluoride (HF) and antimony pentafluoride (SbF5) in equal proportions yields a substance with unparalleled strength.
The result is no ordinary acid. Instead, it’s the most potent acid, or superacid, ever discovered: fluoroantimonic acid, also known as HSbF6.
What Is Flouroantimonic Acid?
Fluoroantimonic acid is a clear, toxic liquid that releases dangerous fumes. Ingesting or breathing it can be deadly, and skin exposure causes severe burns. It reigns supreme among superacids, a class of strong acids far more acidic than sulfuric acid.
Due to its extreme strength, traditional pH or pKA scales are inadequate for measurement. Instead, the Hammett acidity function
Fluoroantimonic acid has an H value of -21, compared to sulfuric acid’s -12. This means fluoroantimonic acid is an astonishing 20 × 10¹⁹ times more potent than pure sulfuric acid.
In a hypothetical face-off, it would resemble Captain Marvel confronting a helpless newborn kitten.
Hydrogen Fluoride and Hydrogen Ions
These elements form the foundation of fluoroantimonic acid, our reigning champion. Acidity stems from hydrogen ions, and their higher concentration amplifies the acid’s strength. While hydrogen fluoride alone isn’t the most powerful acid, it plays a crucial role in creating some of the strongest acids known.
Fluoroantimonic Acid's Kryptonite: Teflon
Despite its unmatched power, fluoroantimonic acid has a critical weakness: it cannot corrode polytetrafluoroethylene (PTFE), better known as Teflon.
As a result, Teflon containers are the preferred choice for storing this highly reactive substance. Alternatively, it can be kept in a hydrofluoric acid solution, which prevents explosive decomposition.
However, caution is essential — this acid can dissolve glass, most plastics, and all organic materials. It also reacts violently with water, causing explosions.
This is far from a simple school experiment. Handling it requires utmost care and should only be attempted by experienced chemists and organic chemistry specialists.
Protonation: Fluoroantimonic Acid's Superpower
Fluoroantimonic acid’s standout ability is protonation — the process of donating protons to organic compounds. This changes key properties such as mass, solubility, and hydrophilicity.
This characteristic is incredibly valuable to chemists, facilitating chemical reactions, glass etching, gasoline refinement, and even the production of explosives. Although it’s recognized as the world’s strongest acid, some argue hydrofluoric acid poses a greater risk because it’s found in everyday products, increasing the chances of accidental exposure.
For those daring enough to handle superacids, wearing personal protective equipment like respirators and safety goggles is essential. This gear acts as a modern chemist’s shield against a substance capable of dissolving flesh and bones instantly.
Comparing to Other Acids
The world of chemistry is expansive, and while fluoroantimonic acid holds a prestigious position, there are numerous other intriguing acids worth discussing. Let’s delve into their unique properties, examining their strengths, limitations, and applications in various processes.
Carborane Acids
These acids rank among the most powerful, second only to fluoroantimonic acid. Their exceptional molecular structure, supported by research from the American Chemical Society, ensures they retain their formidable strength.
What distinguishes them is their weak connection with hydrogen ions, which makes them less destructive compared to fluoroantimonic acid.
Magic Acid
Despite its whimsical name, magic acid is a genuine substance! It’s created by combining fluorosulfuric acid (HSO₃F) and antimony pentafluoride (SbF₅). This acid fully dissociates in water, releasing an exceptionally high concentration of hydrogen ions.
Nitric Acid, Phosphoric Acid and Perchloric Acid
These are strong acids that fully break down when mixed with water. Though they don’t reach the extreme acidity of fluoroantimonic acid, they are indispensable in various industries, from manufacturing fertilizers to powering rocket fuels.
Benzoic Acid and Oxalic Acid
These weak acids only partially dissociate in water. Despite this, they are vital in everyday applications, such as preserving food and serving as cleaning agents.
Hydronium Ion
When acids dissolve in water, they generate this positively charged ion. It’s the true source of a solution’s acidic characteristics.
YouTube’s "Ways to destroy iPhones" is a hilarious category. From smashing them with hammers to dissolving them with substances like gallium, bromine, or fluoroantimonic acid, amateur scientists employ various techniques to challenge the resilience of today’s technology.
